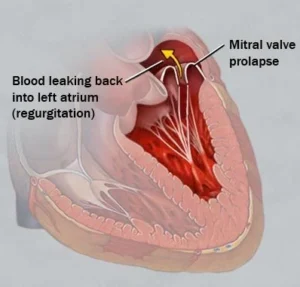
When I was on Fen-Phen back in 1996, I got the Mitral Valve Prolapse it caused and joined in a class action lawsuit regarding the lack of warning for this complication. I got a decent amount of $$ (that paid for the RNY Gastric Bypass a few years later), but I would have prefered to not have my heart deformed by a medication I took.
My mom was in a class action lawsuit regarding the Dalkon Shield in the 1970s and won payments for over a decade. She would have rather not have her uterus shredded by infection with the scary-looking IUD.
It took three years for the Dalkon Shield to be pulled from the market and less than three years for Fen-Phen to be taken off the market.
Thalidomide Birth Defects
Some medications like the anti-nausea medication thalidomide, given to pregnant women around the world in the 1950s and 1960s, took almost a decade of damage before companies recognized the effects and took it off the market.
Thalidomide turned out to change safety rules for medications all over Europe, Australia, New Zealand, and North America. Thalidomide was never approved for use in the United States, but doctors had been given “samples” for their pregnant patients without it having ever been tested on animals or humans. It was assumed to be harmless for pregnant women. Around the world, the medication caused 80,000 to 100,000 babies to die, about 40% at or shortly after birth.
Further, the tragedies associated with this agent stimulated the legislation which revamped the FDA regulatory process, expanded patient informed consent procedures and mandated more transparency from drug manufacturers.

And So Begins a GLP-1 Class Action Lawsuit
I am sleuthing around to find information about the Ozempic class action lawsuit happening in Pennsylvania and the other lawsuits not yet included in the class action case. There are 101 people involved in the Philadelphia case at the moment, but other lawyers around the country are collecting cases they are hoping to add to the class action. The Lawsuit Information Center has updates as they come, so if you’re as curious as I am, follow along.
It seems that the reason for the lawsuits are almost exclusively gastrointestinal. From gallbladder issues to gastroparesis. Although, as you will read, some are a tad outlandish, too.
The Fen-Phen class action lawsuit “…still represents one of the largest monetary rewards for a product liability case in history — over 11,000 plaintiffs filed 6,500 separate lawsuits, but the settlement involved all of the six million users of the Fen-Phen combination.” The payout was $3.75 billion.
The Dalkon Shield class action lawsuit had over 300,000 plaintiffs. $2.5 billion was awarded to those damaged or families of those that died from using the defective IUD.
It is extremely difficult to count the plaintiffs in the Thalidomide scandal because of the number of countries involved and who the payouts went to. All told, about €1 billion were awarded to the victims and the mothers of babies that were miscarried or died. The lawsuits around the Western world took from the 1960s until the 1980s from beginning and end. (Note: I would not use Wikipedia as a reference except this Thalidomide entry has a great deal of information about each of the countries involved. I could not find another source of consolidated information. My apologies.)
GLP-1 Package Inserts

Cherry picking the GI warnings from Mounjaro’s package insert: (emphasis mine)
Inflammation of the pancreas (pancreatitis). Stop using Mounjaro and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your abdomen to your back.
Severe stomach problems. Stomach problems, sometimes severe, have been reported in people who use Mounjaro. Tell your healthcare provider if you have stomach problems that are severe or will not go away.
Gallbladder problems. Gallbladder problems have happened in some people who use Mounjaro. Tell your healthcare provider right away if you get symptoms of gallbladder problems, which may include pain in your upper stomach (abdomen), fever, yellowing of skin or eyes (jaundice), and clay-colored stools.
Common side effects
The most common side effects of Mounjaro include nausea, diarrhea, decreased appetite, vomiting, constipation, indigestion, and stomach (abdominal) pain. These are not all the possible side effects of Mounjaro. Talk to your healthcare provider about any side effect that bothers you or doesn’t go away.
I should have just highlighted the whole thing!
I will share the different GI warnings in Ozempic’s package insert but many of the warnings are the same.

Oh, that’s right. They are all the exact same warnings.
Both have a Boxed Warning (previously called a Black Box Warning) about the risk of thyroid cancer.
If Warnings Are Given, Why Are People Suing?
I have a question before I answer that one.
Why, if people are in pain (mild, moderate, or excruciating), vomiting, can’t eat anything, have bowel impactions, aren’t they calling their providers? Why aren’t they in the hospital? Why are people saying they were in horrible pain for months and couldn’t stop vomiting for weeks and they did not 1) stop taking the medication? 2) GET HELP? Some people stayed on the medication even after they were hospitalized for the issues!
Now, I have read of a few who were hospitalized several times and in and out of the ER. You can’t know how much I would love to see those records. I have a hard time imagining that that many people, using the medications properly, eating decently clean, chewing well, drinking plenty of water, and moving their bodies could have something this serious occur.
And alllll of this in the last year? What about the years between 2017 when Ozempic came out and the year 2023 when the first lawsuit was filed? Not one issue arose? I do understand the numbers game, that so many more people are using the medications now, so complications would be more frequent. However, nothing happened in six years?
Novo Nordisk says, “GLP-1 medicines have been used to treat type 2 diabetes (T2D) for more than 18 years, and for the treatment of obesity for 8 years. This includes Novo Nordisk GLP-1 products such as semaglutide and liraglutide that have been on the market for more than 13 years.”
So why are people suing now? I’m thinking it is something akin to hive mentality. It is so much easier to find people who believe the same things because of the Internet.

But I was in a class action suit, too, though I had medical proof. Not everybody does that are suing about GLP-1s.
In the Daily Mail, quoted below, regarding one of the class action cases in Pennsylvania, one woman is suing for her “deflated breasts.” Another for having her gallbladder removed. Gallbladder issues are a side effect of rapid weight loss. When I had my RNY Gastric Bypass, it was standard practice to remove the gallbladder during surgery. They do not do this anymore, but post-op patients will often have it removed because of problems within a year of their bariatric surgeries.
Let’s Look at One Case
While I am not a lawyer or a doctor, I do have a medical background and I also have common sense. This story also came from the Daily Mail, a UK magazine known for it’s sensational stories that garner hits and a lot of $$.
I’m going to highlight and comment as I go through the paragraphs.
Meredith Hotchkiss, 56, of Idaho, joined nearly 100 patients in a lawsuit against Eli Lilly and Novo Nordisk after she was diagnosed with gastroparesis.
Ms Hotchkiss had been taking Mounjaro and Trulicity, another Eli Lilly injection for type 2 diabetes. She was prescribed Mounjaro from July 2022 until around June 2023. She was also briefly prescribed Trulicity from December 2022 to March 2023.
I’m not quite sure why she was taking two GLP-1s. That is not the standard of care. In fact, stacking, as it is called, is highly discouraged. The woman who died in Australia and made all the news outlets was also stacking.
Please note the length of time she was on the medications. eleven months on Mounjaro and three months on Trulicity… stacking for three months.
Let’s keep going.
Though she has diabetes, her condition is ‘well-controlled,’ so she was given the drugs off-label for weight loss. ‘I thought if I could lose weight and get Mounjaro, then I could try because everybody you see everybody’s doing it,’ she previously told DailyMail.com.
‘The doctor told me that I could lose weight and that it works really well. He said that I would get really sick for four weeks and then after four weeks I’d feel a lot better.’
Within weeks of starting the medications, her condition deteriorated and she couldn’t stomach anything other than cottage cheese, mac and cheese, and yogurt.
Within weeks of starting and she was on Mounjaro for eleven months? And she added Trulicity for three months on top of that? This makes zero sense. What was she thinking? Where was her doctor in this? Did she tell the doctor? Might the doc be the culpable one?
Ms Hotchkiss was fitted with a central line, a tube inserted into the vein to deliver liquid food directly into the bloodstream.
When was the central line put in? Did they take her off the medications? And “liquid food” does not go through a central line (which is placed in a large vein, usually in the chest). Parenteral nutrition goes through a central line, not “liquid food.”
The Cleveland Clinic explains, “Parenteral nutrition is a chemical formula with standard variations and can be customized to your specific nutritional requirements. It may include different amounts of any of the six essential nutrients that your body requires: water, carbohydrates, proteins, fats, vitamins and minerals.”
She (Ms. Hotchkiss) has also been hospitalized three times, including with life-threatening sepsis.
The sepsis (infection) did not come from the GLP-1s she took. Yes, you can get an infection from the central line, but her diabetes can be a factor in getting sepsis. Finding where it started, was it bacterial? Viral? All of these are important aspects to factor in.
And one more thing. Gastroparesis is a complication of diabetes. About 30% of gastroparesis cases are in diabetics, which Ms. Hotchkiss is.
Should the Warnings Include Every Possibility?
A great list of possible effects and side effects from GLP-1s, including hair loss (another common side effect of fast and copius weight loss), Ozempic butt, erectile dysfunction, increased heart rate, dry mouth, and excessive gas can be found on EveryDay Health‘s site in the article, “Every Ozempic Side Effect, Explained.” Should we put every single thing found, even if it might be because of fast weight loss, on the package insert? Should we do that with every medication made in the world?
Let me know what you think.


